Global Market for Intraosseous Infusion Devices


DUBLIN, May 21, 2024 (Globe Newswire) — Intraosseous Injection Devices Market Size, Share by Type (Manual IO Needles, Battery Powered Drivers) and End Use (Hospitals & Clinics, Ambulatory Surgery Centers) and Trend Analysis Report, Forecast by Region and Segment, 2024-2030″ report has been added. ResearchAndMarkets.com Recruitment.
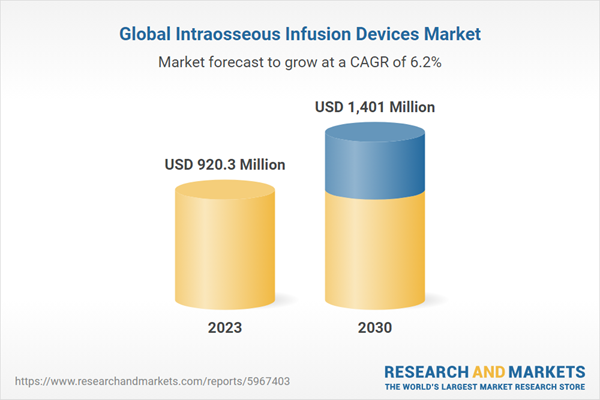
The global intraosseous injection devices market size is expected to reach USD 1.4 billion by 2030 and is projected to grow at a CAGR of 6.2% from 2024 to 2030. This market is growing due to the increasing demand for rapid administration of drugs and fluids directly into the body. These include the vascular system, the rapid increase in the number of surgeries, and the development of technologically advanced intraosseous injection devices. Additionally, the rise in emergency situations such as trauma-related strokes and heart attacks is expected to further drive the market growth during the forecast period.
There are many possible sites for intraosseous injection insertion, including the distal and proximal tibia, sternum, distal femur, and calcaneus. One of the most reliable and fastest means of administering emergency medications to the sternum is intrasternal injection, which provides easy vascular access.


Increased prevalence of cardiovascular disease, increased patient and physician awareness of newly initiated treatments, and large sums of money by governments for advanced technologies adopted for quick and easy administration of drugs, fluids, or blood products. investment. For example, according to the WHO, cardiovascular disease kills 17.9 million people each year worldwide. Furthermore, 4 out of every 5 deaths due to cardiovascular disease are due to stroke or heart attack, and approximately 30% of these deaths occur in the population under the age of 70.
The launch of advanced intraosseous injection devices is estimated to drive the market growth during the forecast period. For example, in June 2023, Teleflex Incorporated received his 510(k) US FDA clearance for the Arrow EZ-1O IO needle for MR conditional labeling. The EZ-IO needle is a key component of the Arrow EZ-IO intraosseous vascular access system. EZ-1O features a diamond tip for accurate, fast, and stable insertion.
Intraosseous Injection Devices Market Report Highlights
-
Based on type, the manual IO needle segment led the market with the highest revenue share of 49.4% in 2023. The most commonly used manual IO needles are the Cardinal Health Illinois /Jamishidi needle, the Cook Critical Care sur-fast needle, and the Cook Dieckman modification.needle
-
Based on end-use, the hospitals & clinics segment led the market with the largest revenue share of 54.9% in 2023. The hospitals & clinics segment is driven by availability of developed and modern infrastructure, increasing investments in developing sophisticated healthcare infrastructure, and an increasing number of skilled professionals.
-
North America dominated the market with a revenue share of 41.33% in 2023. Advanced healthcare infrastructure in the region, growing awareness towards advanced treatments, and increasing aging population contribute to the market dominance.
-
The Asia-Pacific region is estimated to grow at the fastest CAGR during the forecast period due to increased investment in healthcare infrastructure in recent years, especially in emerging countries such as China and India.
Key attributes:
|
report attributes |
detail |
|
number of pages |
110 |
|
Forecast period |
2023-2030 |
|
Estimated market value in 2023 (USD) |
$920.3 million |
|
Projected market value to 2030 (USD) |
$1,401 million |
|
compound annual growth rate |
6.2% |
Main topics covered:
Chapter 1 Methodology and Scope
Chapter 2 Executive Summary
2.1. Market outlook
2.2. Segment Snapshots
2.3. Snapshot of the competitive environment
Chapter 3 Market Variables, Trends, and Scope
3.1. Market system outlook
3.1.1. Parent market outlook
3.1.2. Related/Supplementary Market Outlook
3.2. Market trends
3.2.1. Analysis of market drivers
3.2.1.1. Increased prevalence of cardiovascular disease
3.2.1.2. Increase in the number of emergency patients
3.2.1.3. Rapid increase in approvals and launches of technologically advanced products
3.2.2. Market restraint analysis
3.2.2.1. Restrictions for patients with osteoporosis
3.3. Business environment analysis
Chapter 4 Intraosseous Injection Devices Market – Segment Analysis by Type, 2018-2030 (USD Million)
4.1. Intraosseous Injection Devices Market: Types Dashboard
4.2. Intraosseous Injection Devices Market: Analysis of Type Movements
4.3. Intraosseous Injection Devices Market Size and Trend Analysis, by Type, 2018-2030 ($ Million)
4.4. Manual IO needle
4.5. Battery powered screwdriver
4.6. Impact-powered devices
Chapter 5 Intraosseous Injection Devices Market – Segment Analysis, By End Use, 2018-2030 ($ Million)
5.1. Intraosseous Injection Devices Market: End Use Dashboard
5.2. Intraosseous Injection Devices Market: End Use Behavioral Analysis
5.3. Intraosseous Injection Devices Market Size and Trend Analysis, by End Use, 2018 to 2030 (USD Million)
5.4. Hospitals and clinics
5.5. Ambulatory Surgery Center
5.6. Others
Chapter 6 Regional estimates and trend analysis by country, type, and end use
6.1. Regional Dashboard
6.2. Market size and forecast and trend analysis from 2018 to 2030
Chapter 7 Competitive Environment
-
Aero Healthcare AU Pty Ltd
-
Teleflex Co., Ltd.
-
becton dickinson and company
-
Biopsy Bell SRL
-
cook group
-
SAM Medical
-
Argon Medical Device Co., Ltd.
-
Cardinal Health Inc.
-
Medax SRL Uniperson
-
starfish medical
For more information on this report, please visit https://www.researchandmarkets.com/r/36io1d.
About ResearchAndMarkets.com
ResearchAndMarkets.com is a leading global source of international market research reports and market data providing up-to-date data on international and regional markets, key industries, top companies, new products and latest trends.
attachment
CONTACT: CONTACT: ResearchAndMarkets.com Laura Wood,Senior Press Manager press@researchandmarkets.com For E.S.T Office Hours Call 1-917-300-0470 For U.S./ CAN Toll Free Call 1-800-526-8630 For GMT Office Hours Call +353-1-416-8900