Anti-obesity drugs will be the most influential trend in 2024, followed by personalized and precision medicine, immuno-oncology (IO) drug development, real-world evidence (RWE), and cell and gene therapy (CGT).
This is according to GlobalData’s latest biopharmaceutical update, “State of the Biopharmaceutical Industry 2024 (Mid-Year Update).”
GlobalData is Pharmaceutical Technology.
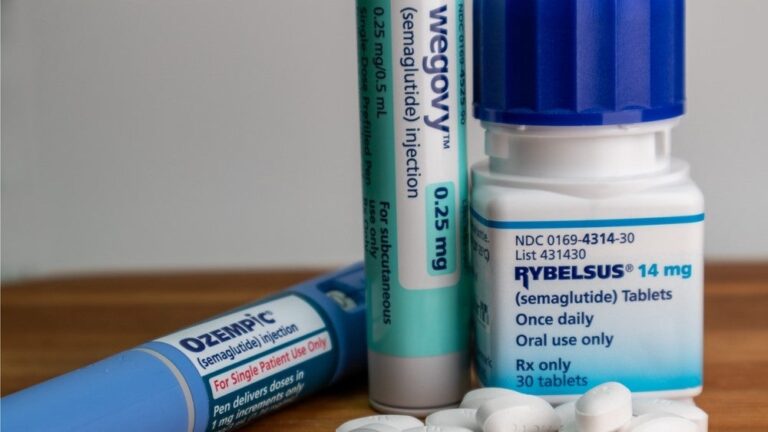
The report notes that the obesity treatment market has seen a surge in popularity since the emergence of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) as a specific drug class. GlobalData predicts that GLP-1 RA market revenues will reach $125.3 billion by 2033, with 90% of this revenue coming from obesity treatments.
The popularity of GLP-1 RAs is spurring R&D investment and driving growth in the metabolic disorders treatment segment, the fastest growing of any therapeutic area. Obesity medications such as Novo Nordisk’s Ozempic (semaglutide), Wegovy (semaglutide) and Eli Lilly’s Mounjaro (tirzepatide) offer better efficacy and safety profiles than other anti-obesity drugs (e.g. appetite suppressants and combination medications), supporting the rapid growth.
The growing market brings opportunity, and pioneers Novo Nordisk and Eli Lilly are already struggling to meet demand. GlobalData points out that the obesity epidemic is here to stay, reporting that “the obese population in the seven largest pharmaceutical markets (US, France, Germany, Italy, Spain, UK and Japan) will grow at an annual growth rate (AGR) of 0.57% over the next decade to exceed 161.5 million in 2033.”
Access the most comprehensive company profiles on the market from GlobalData. Save time on research and gain a competitive advantage.

Company Profile – Free Sample
You will receive a download email shortly
We are confident in the unique quality of our company profiles, but because we want you to make the most beneficial decision for your business, we are offering free samples that you can download by submitting the form below.
From GlobalData
The survey for the report was conducted among 124 GlobalData Pharma clients and prospects between April 5 and May 10, 2024. The survey asked participants to score trends on a scale of 1 to 5 depending on how much they will “impact” biopharma in 2024. Obesity drugs had the highest average score of 4.0, while personalized and precision medicine, IO drug development, RWE, and CGT all received 3.8.
As the most influential trends, 18% of respondents cited anti-obesity drugs, 17% RWE, 8% CGT and personalized medicine, and 6% IO.
The report notes that “CGT, personalized/precision medicine, and IO drug development are closely related trends that have already begun to reshape disease treatment paradigms and healthcare delivery. As more IO drugs and CGTs are introduced to the market, their impact will continue to grow, and the focus on customized disease prevention and treatment will remain a driver and catalyst for value-based healthcare.”
Furthermore, the report predicts that RWE will become increasingly important to both healthcare systems and pharmaceutical companies, with the support of the U.S. Food and Drug Administration (FDA), the Medicines and Healthcare products Regulatory Agency (MHRA), and the EMA.
“RWE is gaining attention as a valuable and necessary source of information to support the decision-making process in healthcare. In the pharmaceutical industry, RWE is expected to play an important role in drug development and approval. With the increasing need to move towards value-based healthcare, more comprehensive and representative research, and more personalized patient treatment, RWE can help facilitate the shift away from volume-based care.”
RWE is increasingly being recognized for its value in clinical trials, providing evidence-based decision-making and improved understanding of drug performance. GlobalData’s Clinical Trials Database shows an increase in RWE studies between 2012 and 2022, and the report cites its potential to improve the efficiency of clinical planning, participant recruitment, study design, and post-marketing surveillance.
Considering the general challenges facing the biopharmaceutical sector, the report concludes: “The pharmaceutical industry often faces regulatory pressure to lower medicines prices and implement stricter control over clinical trials and production standards. However, too much regulation can risk stifling growth and innovation, especially when the industry has to function in an uncertain and difficult economic and geopolitical environment.”